The brand MBST® stands for convincing medical technology. MBST therapy devices combine therapeutic effectiveness and patient comfort with a distinctive design. In everyday practice, they impress with functionality, ease operation, aesthetics and hygienic aspects.
Innovative hightech medical technology
Approved
medical device
TÜV-certified manufacturer
Certified in accordance with EU 2017/45 MDR
MBST® magnetic resonance therapy devices are used for the treatment of indications and types of tissue for afflictions, injuries and painful, degenerative and/or pathological changes of the musculoskeletal system. The tissue-specific treatment on the cellular level allows for a broad range of successfully treated indications. The approved medical devices are exclusively intended for use in medical facilities and centres.
Furthermore, the devices may only be operated by competent staff who have received appropriate user training by an employee or a third party authorised by the manufacturer. This proof of qualification is documented by the manufacturer in the form of a certificate and guarantees the high and consistent quality of MBST treatment worldwide.
The national and international success of MBST therapy is promoted by the ongoing technical development of the therapy systems. Since the market launch of magnetic resonance therapy in 1999, the technology has continuously been further developed always with a focus on effectiveness of the treatment, patient safety and patient comfort. This benefits patients, doctors, clinical facilities and health systems.
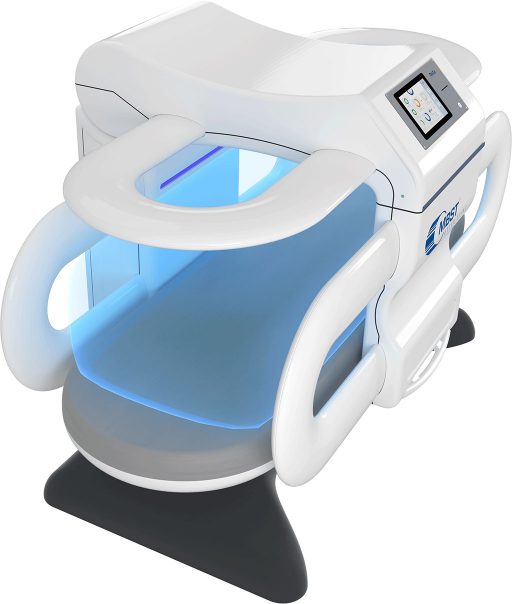
All MBST therapy devices have the same basic technology and active principle. The different device models are optimized for the specific treatment of certain areas of the body, e.g. focal treatment of small joints or whole-body treatment. The current device series SPIN 2 is the third generation of MBST therapy devices, for which we were able to increase field volume and field stability as well as user-friendliness.
Our devices are not mass-produced. Every therapy device and every MBST therapy card undergoes a manufacturing and testing process that is controlled by our quality management system. In this way, our customers and their patients can trust us to deliver and maintain a guaranteed and verifiable standard of our MBST devices at all times.
MedTec was one of the first manufacturers in the medical technology industry to complete certification for our MBST therapy systems in accordance with the strict requirements of the latest EU Directive 2017/45 MDR. This means that our products carry the CE seal with MDR certificate.
The aim of our quality management system in accordance with EN ISO 13485 is to ensure the maximum quality of MBST therapy systems. MedTec products are not mass-produced. Every therapy device and every MBST therapy card undergoes a careful manufacturing and inspection process. This ensures that an MBST magnetic resonance therapy system offers all therapeutic benefits while guaranteeing maximum safety and reliability.
Another essential component is the annual technical inspection of the therapy devices, which guarantees long-term safety, functionality, and ultimately the proper execution of the treatment.
For our customers and their patients, this means that we deliver and maintain a guaranteed and verifiable standard for our MBST products at all times.

The new directive for medical devices has been in force in the EU since 2017: EU 2017/45 Medical Device Regulation (MDR). The MDR places significantly higher demands on medical devices, especially with regard to safety and verifiability of the therapeutic effect. The deadlines have already been extended several times, as not all manufacturers are able to provide the required evidence.
MedTec Medizintechnik GmbH has been meeting the stricter requirements for several years and has already completed the MDR certification process. This means that MBST therapy devices are CE-certified medical devices with MDR approval.
The demanding approval processes in Singapore and Saudi Arabia have also been successfully completed. Certification for the US market in accordance with FDA requirements is currently underway.
This popup shows the full story from each testimonial when “Story lesen” is clicked. You don’t need to edit this text — it will be replaced automatically.